Every chemical that reacts or is soluble or insoluble with another chemical is based on its mutual behavior. My conjecture is that this statement will make most of chemists and chemical engineers ponder “what is the point of this statement/discussion?”. We, humans, are social people who exploit mutual relationships to create excellent affiliations in business, sports and personal adventures. Reality is that chemicals have a mutual behavior and generally it is least understood/exploited in process development. We generally do not exploit and take advantage of their relationships. Having an understanding leads to better economic process and higher quality products (1). In the following I am sharing opportunities I see for excellent processes. Anyone can explore similar opportunities. Perspective presented is my own and not influenced by any external for profit and nonprofit organization.
Due to lack of understanding/exploitation of the relationships quality of the resulting products is assured by repeated in-process and final products analysis i.e. QbA (Quality by Analysis). This is time consuming and expensive effort and lowers profits. It is manifestation of lack of command of the manufacturing process. If the processes were designed such process designers have total command of everything that happens and produces quality product no repeated analysis would be necessary i.e. it will be a QbD(Quality by Design) process. Even with this understanding readers who are more experienced and knowledgeable may be stymied by two alphabets that stand between “A” and “D”. They are “B” bureaucracy (regulators) and “C” concern for not following tradition (2).
Value of taming of interaction and mutual behavior of chemical is incalculable as it has extremely high impact on manufacturing process, product quality and company profitability. Volumes have been written on the subject. Effort can significantly lower solvent emissions per kilo of active pharmaceutical ingredient (API) (3, 4).Through publications attempt has been made share similar perspective (5,6,7,8, 9).
In order to capitalize on mutual behavior of chemicals one has to know and understand chemical and physical properties of every chemical used and produced in each reaction (5,6,7,8, 9). Costs of raw materials and process have to be understood as they dictate the product economics. Familiarity with process equipment used is critical.
Process developers at the laboratory stage do not think and/or exploit chemical and physical properties in the laboratory. Purpose is to develop a laboratory process. Thought of scale-up and commercialization does not enter in the picture at this stage. Conservation regarding a manufacturing process which should begin at inception of the process development but generally is not a consideration (5, 6, 7). Mass balance and mutual behavior of physical properties has to be understood. It is important as they lead to process optimization. Many developers are generally not familiar with manufacturing processes and product costs.
As stated earlier thought of scale-up and commercialization does not enter in the picture at the laboratory stage. Imagination and experience in scaleup of a process can contribute. Involvement of Village (5, 6, 7) from process inception accelerates process development and commercialization. It is an opportunity that is generally ignored but needs to be considered. Following examples illustrate value of physical properties and their mutual behavior. They can be capitalized on to create excellent QbD processes. They have been reviewed (5,6,7,8, 9) and in other publications, too many to cite.
Gabapentin:
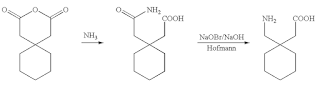
Preparation of Gabapentin is an example of exploiting mutual behavior and physical properties. Its synthesis is illustrated in Figure 1 where mutual behavior of chemicals can be exploited. Review of the filed patent US20080103334A1(10) and other patents suggest solvent use. This is normal for every laboratory process development.
Physical properties of the raw materials (Table 1) suggest that the solvent use can be minimized and/or eliminated. This proposition is based on a process that was commercial about seventy years ago, still in use, to produce a product of similar chemistry at an approximate rate of 1,600 kilos per hour operating about 7,140 hours per year (i.e. about 11,000 MT per year), a continuous process (5,11). Except for water no organic solvent was used.
Global demand for Gabapentin is about 3,135 MT per year (12). One or two plants can produce the product at about 220 kg/hour rate operating bout 7,140 hours per year. However, currently about 70 produce the global demand (13) operating limited hours per year. In their effort they most likely use and generate significant amounts of solvents per kilo of product and even after recovery and re-use have significant impact on our environment. Due to cGMP and cleaning practices asset utilization estimated at 50% on high side (14).
Chemical name | 1,1-Cyclohexanediacetic Anhydride | 1,1 Cyclohexane diacetic mono amide | 1-(aminomethyl) cyclo hexaneacetic acid (Gabapentin) |
CAS number | 1010-26-0 | 99189-60-3 | 60142-96-3 |
Chemical Formula | C10H14O3 | C10H17NO3 | C9H17NO2 |
Molecular Weight | 182.22 | 199.25 | 171.24 |
Melting Point, °C | 70 | 145 -148 | 162-166 |
Boiling Point °C | 126 | 443.6±18.0 predicted |
|
Table 1: Properties of chemicals used and produced in Gabapentin
Process that is similar to the chemistry in Figure 1 had the anhydride fed as a melt with stoichiometrically controlled addition of liquid ammonia. Use of liquid ammonia eliminated water as a solvent. Melt acted as a solvent for the next step (5). Resulting amide was reacted with in-situ produced chlorine and feed stoichiometrically. Intermediate produced was converted to the product by stoichiometrically controlled acidification. Details are not discussed.
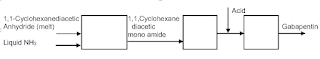
Continuous conversion of 1,1-Cyclohexanediacetic Anhydride to 1,1 Cyclohexane diacetic mono amide to produce Gabapentin would be an excellent and simple process. Its processing conditions will have to be optimized and can result in an excellent continuous process. Figure 2 is conceptual manufacturing process scheme for Gabapentin. Similar chemistries have been commercial. As stated earlier details are not discussed.
Figure 2: Continuous Gabapentin Manufacturing Process
In the proposed continuous process 1,1-Cyclohexanediacetic Anhydride would be metered as a liquid along with liquid ammonia in required stoichiometric ratio. Formed amide would be converted to Gabapentin using chlorine based bleach that will be produced inline and fed to the reaction system. Chlorine based bleach is significantly cheaper than bromine based reactant. Bleach will have enough water to slurry the product for subsequent processing. The proposed process would need necessary development work to have an optimum process. Pfizer did attempt to green its batch process (15).
Process for Piperine Intermediate:
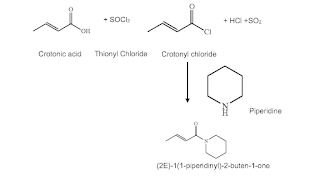
Discussed below is an example two steps for the process to produce Piperine (16) intermediate, Figure 3. Chemistry and physical properties suggest that the first two steps could be a solventless process. Any astute chemist and chemical engineer should consider all liquid process to produce (2E)-1(piperidinyl)-2-buten-1-one which is a liquid.
Figure 3: First two steps of USP 11,267,798 B2 (16)
Chemical Name | Crotonic acid | Thionyl Chloride | Crotonyl chloride | Piperidine | (2E)-1(1-piperidinyl)-2-buten-1-one |
CAS Number | 107-93-7 | 7719-09-7 | 10487-71-5 | 110-89-4 | 3626-69-5 |
Chemical Formula | C4H6O2 | SOCl2 | C4H5ClO | C5H11N | C9H15NO |
Molecular Wt. | 86 | 119 | 104.5 | 85 | 153 |
Melting Point, °C | 70-73 |
|
| -7 | (-ve) 3-5 |
Boiling Point, °C | 185 | 76 | 121-123 | 107 | ~295 |
Table 2: Properties of chemicals used and produced in USP 11,267,798 B2 (15)
Once the chemical physical and chemical properties of the reactants and intermediates are understood, it is very feasible to create a safe and simple commercial process. In every reaction step solubility and insolubility of chemicals used matters and attention has to be paid as it can lead to significant process optimization and reduction of solvent use leading to “Net Zero (3, 4)”.
Most of the fine/specialty chemical and their subset API plants have sufficient idle equipment that can be assembled for batch or continuous processes. Creativity and imagination and Village’s (5, 6, 7) support would be needed. Yields of many products could be higher (9,17) and processes can be simplified. Effort will be necessary for innovation and better methods.
If what all has been discussed (5, 6, 7) above is applied for product and process development any product that has an annual demand of about 50 to 100+ Metric Tonnes per year per site can be produced using a continuous process (5, 11). A concerted effort would be needed. Solvent use could be reduced and overall productivity improved. Chemists and chemical engineers have to exploit and apply all of their knowledge and learning. Process design will require every bit of creativity, imagination to capitalize mutual behavior of chemicals used and produced (5, 6, 18, 19, 20, 21). Using the methods reviewed above batch processes for new brand and generic products can be optimized along with lower commercialization time and will have lower “Net Zero” (3,4) impact than the current batch processes. Judicious ROI analysis will have to be done. Another benefit of such designs will be that product demands can be addressed very quickly resulting no or minimum shortages.
As suggested earlier incorporation of the suggested/proposed methods is not going to easy. Traditions of the last seventy plus years used to manufacture API will come in the way. Desire to change has to come from within each company and has to be acceptable to the regulators who are not familiar with discussed chemical synthesis methods and manufacturing practices. My hope is that simplicity of processes and improved asset utilization will lead to consistently superior quality products and higher profits will be the driver for their adoption. Time will be the spokesperson.
Girish Malhotra, PE
EPCOT International
1. Malhotra, Girish: Sociochemicology May 30, 2013 Accessed January 13, 2023
2. Malhotra, Girish: Alphabet Shuffle: Moving From QbA to QbD - An Example of Continuous Processing, Pharmaceutical Processing, February 2009 pg. 12-13
3. Burke, J. What does net zero mean? https://www.greenbiz.com/article/what-does-net-zero-mean, May 2, 2019 Accessed April 27, 2021
4. Sheldon R.A. The E factor 25 years on: the rise of green chemistry and sustainability, Green Chemistry https://pubs.rsc.org/en/content/articlelanding/2017/gc/c6gc02157c/unauth#!divAbstract , 2017, 19, 18-43 Accessed February 17, 2021
5. Malhotra, Girish: Active Pharmaceutical Ingredient Manufacturing: Nondestructive Creation De Gruyter April 2022 Accessed May 24, 2023
Malhotra, Girish: Chemical Process Simplification: Improving Productivity and Sustainability, John Wiley & Sons, February 2011 Accessed May 24, 2022
7. Malhotra, Girish: Chapter 4 “Simplified Process Development and Commercialization” Quality by Design-Putting Theory into Practice co-published by Parenteral Drug Association and DHI Publishing© February 2011 Accessed May 24, 2022
8. Malhotra, Girish: Profitability through Simplicity June 15, 2023 Accessed May 24, 2023
9. Malhotra, Girish: Considerations to Simplify Organic Molecule (API) Manufacturing Processes: My perspective, Profitability through Simplicity April 20, 2019 Accessed May 24, 2023
10. Kumar, A. et. al. IPCA Laboratories Process for Synthesis of Gabapentin USP 20080103334A1, Published May 1, 2008 Accessed June 6, 2023
11. Continuous Production https://bit.ly/2Rp3Xlu Accessed June 5, 2023
12. Kumar, S. Top Ten Most Produced API in India https://www.linkedin.com/posts/apibusinessanalyst_top-10-most-produced-apis-in-india-in-2022-activity-7068750309526781953-frPN?utm_source=share&utm_medium=member_desktop Accessed June 1, 2023
13. Gabapentin Indian API Producers https://www.pharmacompass.com/active-pharmaceutical-ingredients/gabapentin, Accessed June 1, 2023
14. Benchmarking Shows Need to Improve Uptime, Capacity Utilization, https://www.pharmamanufacturing.com/articles/2007/144/ Sep 20, 2007, Accessed May 19, 2020
15. Liam Tully, Green API Manufacturing, Pharmaceutical Technology, Volume 33, Issue 9, pp. 46-48 Accessed December 9, 2009
16. Phull M.S. et. al. Process for the Preparation of Piperine US 11,267,798 B2 March 8, 2022 Accessed June 12, 2023
17. Malhotra, Girish : Capitalizing on Mutual Behavior and Chemical Reactivity of Chemicals, Profitability through Simplicity, May 29, 2023 Accessed June 6, 2023
18. Malhotra, Girish: Considerations to Simplify Organic Molecule (API) Manufacturing Processes: My perspective, Profitability through Simplicity, April 20, 2019 Accessed June 5, 2023
19. Malhotra, Girish: Review of Continuous Process for Modafinil, Continuous Processing in the Chemical and Pharmaceutical Industry II, 2009 AIChE Annual Meeting, November 10, 2009,Nashville, TN.
20. Malhotra, Girish: Analysis of API (Omeprazole): My perspective, Poster Session: Pharmaceutical Engineering, 2009 AIChE Annual Meeting, November 11, 2009, Nashville, TN.
21. Malhotra, Girish: Art and Science of Chemical Process Development & Manufacturing Simplification, AIChE.org May 17, 2023 Accessed June 10, 2023

No comments:
Post a Comment